Abstract
Fanconi anemia (FA) is a genomic instability disease caused by defective DNA repair which predisposes patients into developing myelodysplastic syndrome (MDS) or acute myeloid leukemia (AML). Identification of clonal abnormalities in FA patients is vital to detect transformation before the onset of a high-grade MDS/leukemia.
We analyzed bone marrow (BM) samples from a series of 50 patients with FA who were diagnosed at the Christian Medical College, Vellore, India from January 2005 - July 2017 by peripheral chromosome breakage analysis (CBA) or FANCD2 ubiquitination analysis of peripheral blood or dermal fibroblasts. Inclusion in the current study was based on the availability of karyotyping samples performed on the BM aspirate collected at diagnosis. Peripheral blood and dermal fibroblasts were collected at diagnosis for CBA and FANCD2 western blot.
All 50 patients evaluated had phenotypic features suggestive of FA. There were 28 patients who had increased CBA scores alone while 21 had both increased CBA scores as well as defective FANCD2 ubiquitination. One patient with negative CBA score showing hematopoietic mosaicism with defective FANCD2 ubiquitination pattern in dermal fibroblasts alone was also included in the study.
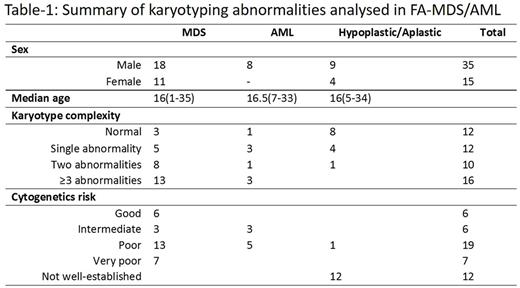
The median age at diagnosis was 16 years (range 1-35) and 35 (70%) were male. Abnormal karyotypes were obtained in 38 patients while the remaining (n=12) showed a normal karyotype. Examination of bone marrow aspirate and trephine was consistent with MDS in 29 (MDS-RS-MLD n= 3; MDS-EB n= 10; MDS-U n=12; h-MDS n=4), while the remaining had either AML (n=8) or a hypoplastic marrow (n=13). The 12 patients who had a normal karyotype were predominantly hypoplastic (n=8) or had either MDS (n=3) or AML (n=1). Abnormalities of chromosomes 1 and 7 were the most commonly reported chromosome aberration and was seen in 17 patients each (45%). A partial deletion 5q was present in 7 (18%) patients while gains of chromosomes 8 and 21 were seen in 5 and 6 patients respectively. Complex karyotypes involving abnormalities of 3 or more chromosomes were seen in 16 (42%) patients who had MDS/AML. FA gene mutation analysis of 8 patients showed FANCA gene mutations in 7 patients (4-abnormal KT, 3-normal KT) while 1 patient with abnormal karyotype had a mutation in the FANCJ gene.
Post treatment karyotypes were available in 2 patients with AML and 1 patient with MDS which showed persistence of the leukemic clone identified at diagnosis (n=1) or clonal evolution (n=1, gain of chromosome 9) while the third patient showed a new clone after treatment which retained two of the three abnormalities documented at diagnosis.
Using the Revised International Prognostic Scoring system for MDS majority of the patients (n=20) with MDS were classified in the poor or very poor risk groups while the remaining patients were in the good (n=6) or intermediate (n=3) risk group.
Five of the 8 patients with AML were classified as poor risk according to the refined MRC cytogenetic classification while 3 were grouped in the intermediate risk group.
Among the 13 patients showing a hypoplastic marrow, one patient had monosomy 7 which has been shown to have an increased risk of transformation to MDS/AML. One patient who had gain of 1q due to an unbalanced translocation transformed to AML in 9 months.
We report a distinct pattern of chromosome abnormalities in patients with FA-MDS/AML consistent with previous reports, underscoring the need for inclusion of karyotype analysis in the laboratory evaluation strategy of FA patients for early detection of transformation to hematologic malignancies.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal